iCFree
Improved cell-free systems to accelerate therapeutic proteins production at minimal cost


Funded by one of the 5 grand challenges launched by the PIA program of the French government Biomedicines: improving yields and controlling production costs
Healthcare systems are increasingly dependent on access to critical, high-value proteins, which are often environmentally damaging and complex to obtain domestically. The emergence of functionalized and customized proteins is enabling a radical transformation of therapies, which are becoming more individualized and complex. Nevertheless, the production costs of new biological drugs are skyrocketing compared to chemical drugs. These costs are also very susceptible to the technology used and the production batch size. As far as cell and gene therapies are concerned, these techniques are not yet fully industrialized and optimized. To meet these needs, progress is necessary. Cell-free systems have lately revolutionized the field of synthetic biology as they provide a fast and robust platform for protein production.
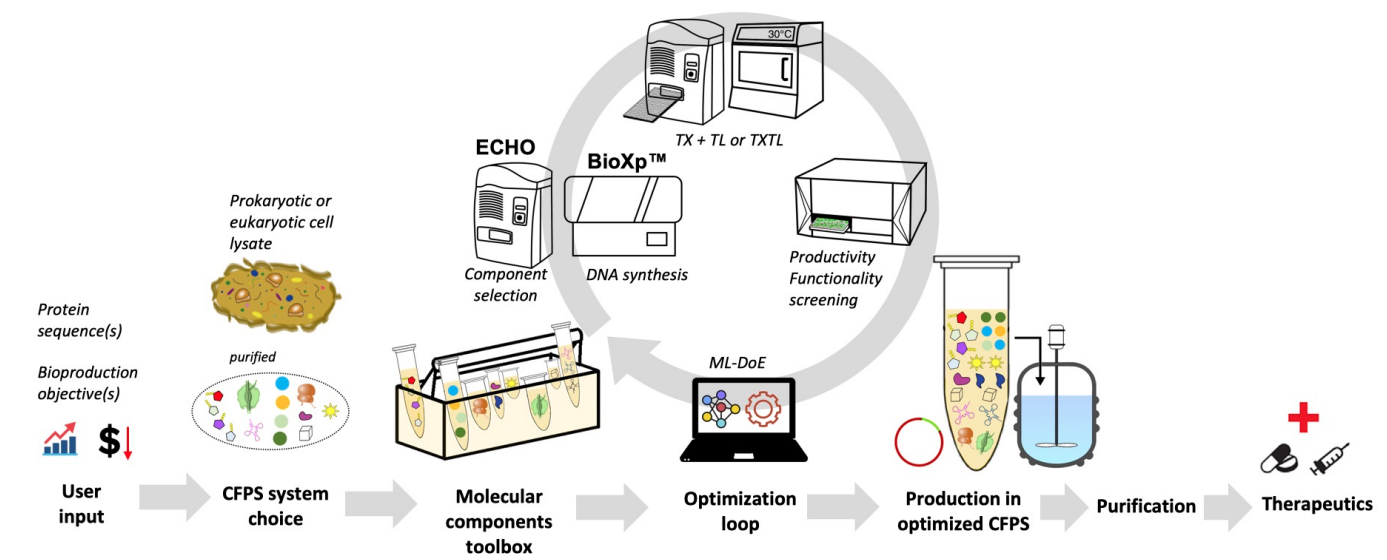
iCFree aims to develop and optimize cell-free protein synthesis systems (CFPSs) to produce therapeutic proteins which are difficult to synthesize in vivo. The project uses machine learning tools to develop and optimize CFPSs. To reach the commercial market for protein production, CFPSs must evolve from a laboratory level to a robust production platform. To that end, several factors need to be optimized, like the synthesis of high-quality functional proteins with proper folding and post-transcriptional modifications, the cost of production, and the scalability. Addressing these factors requires more detailed understanding of the CFPSs components to optimize lysate quality, the reaction conditions, and the buffer elements.
Recently, we proposed a strategy based on machine learning to explore large combinatorial spaces (~4,000,000 cell-free buffer compositions) varying several components at one time to efficiently find an optimum for cell-free productivity. That latter work demonstrated that when compared to a standard cell-free preparation protocol (Sun, Zachary Z et al), protein productivity was increased by 34 folds, and cost was reduced by lowering buffer components concentrations. Here we will expand upon that strategy optimizing for the multi-objective of maximizing productivity and minimizing cost while maintaining functionality. For the three proposed CFPSs, an optimization machine-learning-based workflow (image below) will help determine the stoichiometry of specific supplementary components such as chemicals, enzymes, DNA, tRNAs, or riboproteome. Provided a gene sequence, within less than 10 days the systems will enable on-the-fly synthesis, transcription-translation of the gene, quantification-purification of the corresponding protein for further scale up or small-scale on-site and on-demand production at point-of-care facilities.